24 May ARTICLE “PHARMACOVIGILANCE KEY CONCEPTS”
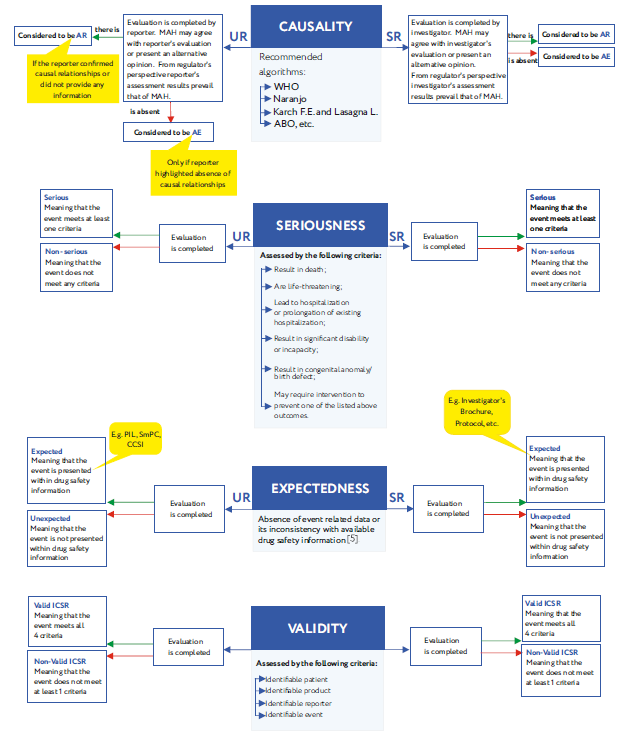
Key words: Patient information leaflet (PIL), adverse reaction (AR), adverse event (AE), company core safety information (CCSI), summary of product characteristics (SmPC), unsolicited (spontaneous) reports (UR), solicited reports (SR), clinical trials (CT).
Pharmacovigilance is a complex system that allows all parties involved in drug circulation to ensure medicinal products safety and efficacy [1]. One of pharmacovigilance key elements is various data related to the mentioned issues (e.g. adverse reactions and events, drug interactions information). The exchange of such data is a complex and multi-stage process, the efficiency of which is largely dependent on marketing authorization holder (MAH) performance.
Please see the full text to Pharmacovigilance key concepts 24 May 2018



No Comments