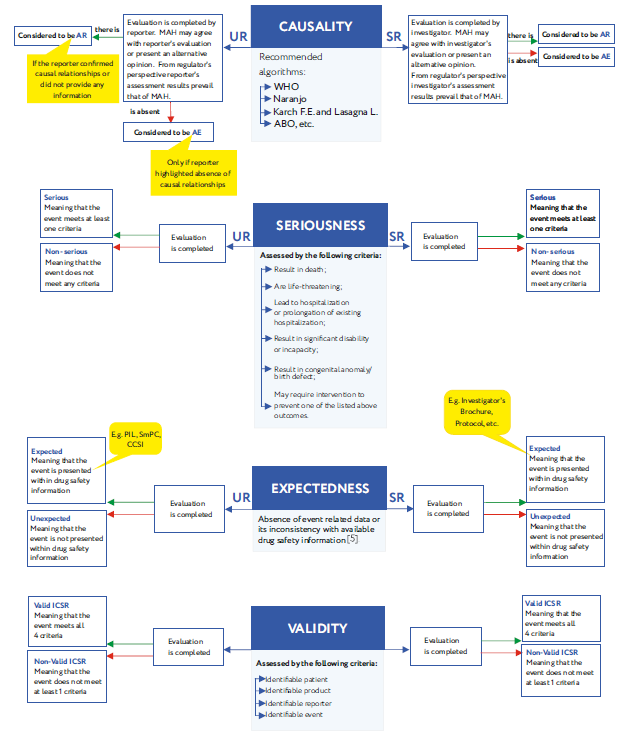
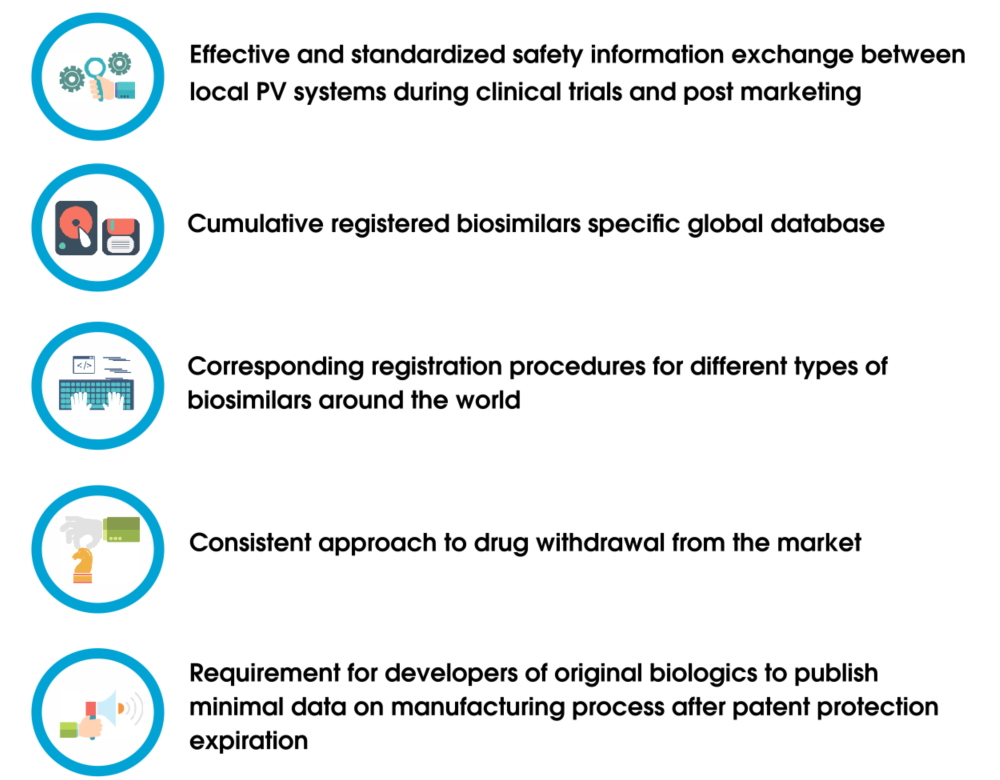
Guidance to the pharmaceutical industry during the COVID-19 pandemic_updated news
Due to the current epidemiological situation, the issue of developing and implementing additional measures to ensure the safety of participants in current and planned clinical trials, as well as consumers of medical products within the framework of post-marketing surveillance has become more relevant for the......