Established 2004
Our History
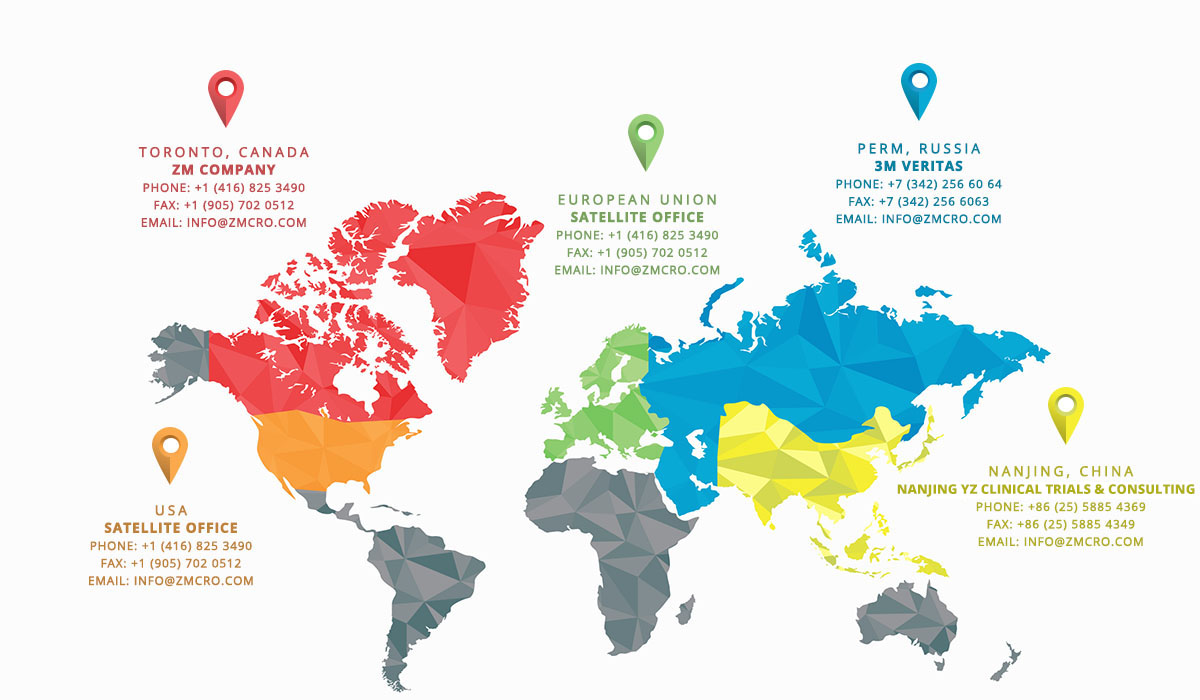
In 2004, ZM Company started as a medical review and drug safety boutique consultancy CRO in Canada. In 2014 we have evolved into an international organization with offices in China, Russia, satellite offices in USA and EU, providing our clients around the globe with top-quality specialized drug safety services in all types of clinical trials (including Phase I-IV, Proof-of-Concept, Post-Marketing Research and Surveillance). ZM Company has established relationships with trusted partnering companies, and has a full-service CRO capabilities on international arena.
The Mission

Facilitate pharmaceutical, biotechnology and medical device companies in improving global healthcare by providing creative expert solutions for their projects.

Exploit our experience, knowledge and creative approach to improve care for patients.

Establish long-term partnering relationship with clients by providing services with professionalism and dedication.

Support our clients in bringing their drugs and/or device to the market in a fast and efficient manner.

Invest our knowledge, expertise and creativity in improving clinical trials conduct.
Values
Expertise
Apply global experience and knowledge to find optimal solutions.
Team
Support and communication to open new prospects for development.
Ethics
Priority is given to data confidentiality & integrity, patients & clinical trial safety when planning and performing the project.
Excellence
Adhere to world highest standards of quality to meet clients’ and regulatory requirements.
Clients
Focus on client needs and requirements to provide best possible results and establish long-term partnering relationships.
Careers
One of the ZM Company’s key priorities is its employees’ wellbeing. Our company is committed to creating comfortable working environment that would help to develop your professional skills and personal qualities.
We ensure that all company employees constantly learn and advance by participating in conferences and trainings, attending international and local seminars and workshops.
ZM Company offers wide range of career opportunities, exciting meaningful professional challenges and welcomes specialists with medical, pharmaceutical, biological backgrounds with and without practical experience in clinical trials.
If you are interested in joining our team, please forward your resume to info@zmcro.com
Confidentiality Policy
To view our Confidentiality Policy please follow to this link BP06-POL-19 Confidentiality Policy 1.0 01Mar2024